

If the electron is bound to a nucleus of arbitrary charge +Z, then the energy of the electron is The nucleus of a hydrogen atom has a charge of +1. The energy of the electron in a hydrogen atom depends only on the principal quantum number, n. It should be noted that the subshells are energy levels as well, called Subsidiary orbital energy levels, so if we sort the subshells in ascending order in terms of their energy levels, it would be s < p < d < f. To denote the value of 'l' instead of 1, 2, 3… some spectroscopic symbols are used.
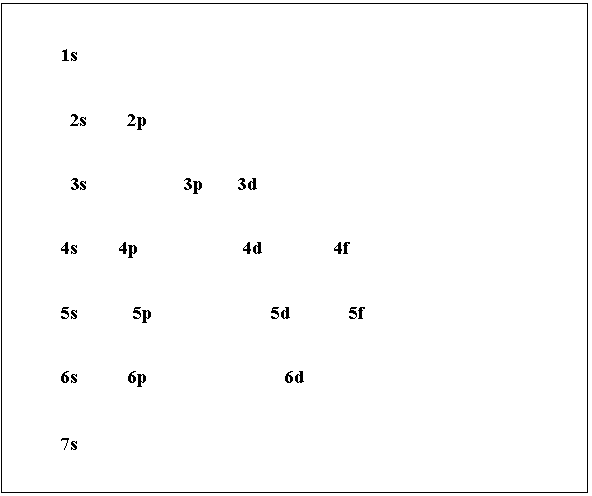
The Azimuthal or Subsidiary quantum number helps to determine the ellipticity of the subshells. He imagined that other than the circular orbits that Bohr established, there are elliptical orbits as well. What is the Azimuthal Quantum Number (L)?Īrnold Sommerfeld delved deeper into the orbitals chemistry, he viewed every orbital energy level or shell is made up of many subshells. For example, for the K-orbital n = 1, for L-orbital n = 2, for M-orbital n = 3. A Principal Quantum number is denoted as 'n'. These numbers are the Principal Quantum Numbers. The Orbitals are named K, L, M, N… or 1, 2, 3, 4… in ascending order. The foundation of orbitals chemistry starts with Bohr who established that electron orbitals represent an energy level in terms of their distance from the Nucleus. Hence, the electrons in the different shells which are at different distances have different energies. The electron is bound more tightly to the nucleus if the electron is closer to the nucleus. Therefore, the force of attraction between an electron and its nucleus is directly proportional to the distance between them. Still, the perk here is it can give the fundamental understanding of the Nucleus posing as the Sun at the center and the Electrons orbiting the Nucleus matching planets with the disparity that the Electrons populate the regions of space, which are called the Orbitals and the orbitals have varied orbital energy levels.Īs the magnitude of the changes increase, the magnitude of force also increases, and the forces decrease when the separation of charges is more. The idea of the atomic structure much like a solar model is flawed in many ways. As the study in the field of chemistry furthered over the centuries, scientists preferred rationalizing the otherwise anomalous behavioral patterns of atoms and molecules, thus introducing the Periodic Table put forward by Mendeleev and the Atomic Structure by Ernest Rutherford, later corrected by Bohr.


 0 kommentar(er)
0 kommentar(er)
